Radiotherapy for Head and Neck Squamous Cell Carcinoma State of the Art and Future Directions
Abstruse
During the by 20 years, treatments for head and cervix squamous cell carcinoma (HNSCC) take changed dramatically attributable largely to the appearance of novel approaches such as combined modality therapy likewise as improvements in surgical and radiotherapeutic techniques. Locally advanced affliction in particular, which engendered very high recurrence and mortality rates, is now associated with long-term disease-free survival in the majority of cases. This article volition focus on locally avant-garde HNSCC, which frequently remains a clinical challenge, review country-of-the-art therapy, and introduce promising novel therapies. The field continues to evolve rapidly with new testify during the past year clearly establishing the benefit of adjuvant chemoradiotherapy (CRT), equally well every bit early evidence showing improved survival with the use of an epidermal growth factor receptor inhibitor in combination with radiotherapy. At that place are varied regimens in use for patients with locally advanced disease, but at the aforementioned time the multitude of options can plague the clinician when trying to select the most appropriate one. This article will try to put the various approaches into perspective and propose an evidence-based treatment algorithm.
Principal
Head and cervix cancer has an estimated annual global incidence of 533 100 cases (Parkin et al, 2001) and is the fifth most mutual cancer worldwide with the great majority of cases beingness squamous prison cell carcinomas (HNSCC). There is strong geographical variance in incidence probable related to associated risk factors, with the highest reported rates being observed in some areas of France (Bas-Rhin, male person incidence 63.58 cases/100 000 people) and India/key Asia (Sankaranarayanan et al, 1998). The staging for HNSCC is shown in Table i. Treatment for locally advanced affliction (stages Iii, IVA, IVB), which makes up more than than l% of all cases, requires aggressive and concerted measures, and frequently remains a clinical challenge. Until recently, v-year survival rates were reported to exist below xxx% for patients with stage IVA/B disease (Vokes et al, 1993) and 40% for all locally advanced tumours (Laramore et al, 1992) even with early multimodality approaches (30% recur locally, 25% distally).
Perspective
Current direction of locally advanced HNSCC (Tabular array 1) has evolved from poorly effective single modality therapy to an integrated, highly constructive multidisciplinary approach. Unlike early on phase HNSCC, all three modalities – surgery, radiotherapy, and chemotherapy – play vital and complementary roles. The diverse combination treatments have led to several competing approaches, each with distinct advantages and disadvantages, and initial treatment can vary significantly between institutions. Therefore, the post-obit paragraphs will examine each major approach, highlight important differences and advantages/disadvantages, and try to recommend a management flowchart based on the evidence. Treatments used for locally advanced disease tin be classified every bit follows:
- i
Surgery followed by adjuvant chemoradiotherapy (CRT) (or radiation)
- two
CRT upfront (with surgery every bit a salvage treatment)
- iii
Induction chemotherapy followed by definitive local therapy:
- a)
CRT
- b)
Other principal treatment options: radiotherapy, surgery (±adjuvant therapy)
- a)
- iv
epidermal growth factor receptor (EGFR) inhibition in combination with radiotherapy or CRT
Postoperative therapy: surgery followed by adjuvant CRT (or radiation)
Predictors of recurrence after surgical resection include involved margins of resection, extranodal/extracapsular spread, perineural invasion, and the presence of 2 or more than involved regional lymph nodes (Snowfall et al, 1982; Vikram et al, 1984; Kramer et al, 1987). Since locoregional failures remained the dominant trouble, adjuvant locoregional therapies such as radiation and subsequently CRT were added and adjuvant therapy is now considered standard of care for stage 3/IVA/B disease.
Adjuvant (postoperative) radiotherapy is well studied (Fletcher and Evers 1970; Snowfall et al, 1982; Vikram et al, 1984; Kramer et al, 1987). It decreases local failure rates and although only retrospective bear witness exists (Huang et al, 1992), there is broad consensus since the 1980s that it increases survival and a randomised prospective trial would be unrealistic at this point. Still even with adjuvant radiotherapy, in the presence of high-take a chance features, the adventure of local recurrence (27–61%), distant metastases (xviii–21%), and decease (five-year survival charge per unit 27–34%) remain unsatisfactorily high (Cooper et al, 1998).
Postoperative (adjuvant) CRT offered an arroyo that could heighten local control with radiosensitising chemotherapeutic agents. Several studies take demonstrated that concurrent CRT is a highly effective therapy for locally advanced HNSCC including tumours that are not amenable to surgery (El-Sayed and Nelson 1996; Brizel et al, 1998; Vokes et al, 1999; Jeremic et al, 2000; Adelstein et al, 2003; Vokes et al, 2003), justifying trials of concurrent CRT as postoperative (adjuvant) treatment.
The first trial to suggest a marked do good of postoperative CRT over radiation lonely in patients with locally advanced disease with loftier-run a risk features was a smaller trial by Bachaud et al published in 1996 (Table 2a) (Bachaud et al, 1996). In club to ostend the Bachaud trial, ii similarly designed, multicentre, randomised stage Iii trials of adjuvant radiation vs CRT were reported in 2004 (Bernier et al, 2004; Cooper et al, 2004) also in patients with stage III, IVA/B disease and high-risk features for recurrence. The RTOG 9501 and the EORTC 22931 (summarised in Table 2) were able to produce level 1 evidence of a articulate benefit for adjuvant CRT, at the toll of a pregnant increase in acute toxicities. Inclusion criteria and the type of radiotherapy varied somewhat between the two trials (Table ii), but overall they were coordinated to employ similar treatment protocols, making the results appear very robust. Both trials support the benefit of CRT over radiations alone in patients with high-adventure features. Although only the EORTC trial showed a significant survival reward for CRT, the RTOG trial trended in the same direction and showed a significant increment in progression-free survival.
Likewise consistent in both trials was an increase in acute class Iii and Iv toxicities in the combined therapy arm (Table 2) including toxic deaths. On the other mitt, at that place was no difference in astringent long-term handling-related toxicities. Strong consideration should, therefore, be given to handling of these patients in centers with expertise in combined modality treatment and a well-established supportive care system. A positive correlation of treating institution expertise and patient survival supports this belief (Benasso et al, 1997).
Although we can conclude that postoperative CRT in patients with locally advanced disease with high-chance features is now standard of care (Tabular array 2a), for patients without high-risk features, the testify of a benefit of CRT over radiation lone is less clear with no randomised trials addressing this question. The Intergroup trial 0034 (Laramore et al, 1992) (see Tabular array 2b) may give some insight, simply clearly was non intended to address this particular question equally it used sequential chemotherapy and radiotherapy in comparison to radiations alone. This trial did enroll a significant portion of intermediate take chances patients (phase Three illness) without loftier-adventure features and did non find a difference in local command, disease-costless, and overall survival. Interestingly, distal failures were decreased presumably due to the higher doses of systemic chemotherapy compared with concomitant CRT. Despite the enrollment of lower take a chance patients, overall local control rates were lower than in the more than recent EORTC and RTOG trials. These trials were reported 12 years apart and make comparisons difficult due to stage migration and full general improvements in supportive care and therapeutic modalities.
Even though adjuvant concomitant CRT as reported in these two landmark trials is a major step, information technology needs to be noted that the locoregional failure rate remains unsatisfactorily high at thirty%. Attempts have been made to farther improve the radiosensitising properties of chemotherapy using doublet and triplet combinations (see beneath) with the hope of further improving survival. Many centres have adopted this approach, based on stage 2 evidence indicating safety, feasibility, and potentially improved efficacy, but this remains an unanswered question until phase III data become available.
The optimal fourth dimension frame to start adjuvant treatment postsurgery has non been studied sufficiently. Limited evidence and clinical experience with the fourth dimension needed for patients to recover advise that it should be inside four–six weeks postsurgery.
Definitive/concomitant CRT and organ preservation
During the by decade, an attractive alternative to initial surgery has evolved. Originally pioneered for inoperable patients, upfront concomitant CRT has emerged as a definitive treatment selection comparable to upfront surgical management in resectable patients. Given its advantage with regards to organ preservation and excellent reported local control and survival rates, CRT is increasingly used and has become the dominant treatment modality in many centres (Hoffman et al, 2004). The decision betwixt upfront surgery followed by chemoradiation vs upfront chemoradiation with the option of save surgery remains controversial and depends on many factors, including local expertise, goals for organ preservation, operability, resectability, and patient preference. No adequate randomised trial has examined this question and, given inherent biases in patient pick and power to stage patients in a comparable fashion, it is unlikely that we will have a definitive answer in the about future. Both approaches piece of work well, can coexist, and allow matching of treatments to a patient's disease and preferences.
Concomitant CRT
Concomitant CRT attempts to capitalise on radiosensitising backdrop while delivering systemically active agents. Sensitising effects though are not selective for neoplasm cells, and next normal tissue inside the field is also subject to more than effective and more toxic radiation. Consistently, CRT trials study an increased incidence of form 3 and iv acute toxicities with mucositis and dermatitis existence the most prominent. On the other hand, astringent long-term side effects are not increased in comparison to radiation lonely, and nigh all patients recover from the intense treatment. As mentioned, handling should preferentially be done at experienced centres that accept an advisable support infrastructure (Benasso et al, 1997).
Multiple phase Two trials using intensive CRT regimens take shown long-term survival rates of 60–70%, without surgery, for locally advanced HNSCC (Kies et al, 2001; Adelstein et al, 2002; Machtay et al, 2002; Vokes et al, 2003). Phase Ii trials need to be interpreted with circumspection due to their inherent biases, but the consistency of the results and the big number of patients treated with this arroyo is reassuring. Meta-analysis of early on randomised, only generally underpowered, trials suggested an accented survival benefit of approximately 8% at 5 years for CRT over radiation also (NNT=13; 13 patients need to be treated to salvage i life) (El-Sayed and Nelson, 1996; Pignon et al, 2000). More contempo trials furthermore propose fifty-fifty more robust survival benefits with an absolute take chances reduction of death at 3 years of 14–25% (NNT=4–7; betwixt 4 and 7 patients demand to be treated to salve one life) (Brizel et al, 1998; Adelstein et al, 2003).
Based on suggestive phase Two bear witness, contempo trials often at present investigate combination chemotherapy. Commonly used agents include cisplatin, 5-FU (Table ii), taxanes, hydroxyurea, and gemcitabine (Vokes et al, 2003; Milano et al, 2004). Concomitant CRT is among the near efficacious locoregional control measures, but at the same time, these contempo trials reveal a shift in the pattern of failure towards afar disease especially in patients with advanced nodal stage. Induction chemotherapy attempts to subtract distal failures has been advocated now that local control is achieved in nearly patients (encounter below).
Even though some controversy remains, at that place is an increasingly meliorate divers part for surgical management of certain patients afterwards CRT (Argiris et al, 2004). Cervical lymph node autopsy (ND) fifty-fifty after a complete response (CR) to CRT is advisable in patients with N2-N3 illness to optimise locoregional illness control (Lavertu et al, 1997; Argiris et al, 2004). Too, all patients with residual lymph node enlargement on imaging should undergo ND, even though many specimens volition only show necrosis. In patient with higher LN status (N2 and higher), upwards to 35% of specimen volition harbour residue microscopic tumour (Stenson et al, 2000). In a contempo trial, this arroyo was able to improve progression-free survival (Argiris et al, 2004). In contrast, patients with N0-1 disease and a CR to treatment did not benefit. A selective lymph node dissection is feasible four–12 weeks after CRT and is associated with an excellent safety profile (Stenson et al, 2000).
Organ preservation
Given that concomitant CRT increases locoregional control, and thereby avoids surgical resection of important anatomical structures, it was postulated that CRT may offer superior organ preservation in comparison to surgery, radiation, or sequential chemotherapy and radiation. Initially, two trials using sequential chemotherapy and radiation in comparison to surgery with adjuvant radiations reported improved organ preservation with no difference in survival (Table three) (VA Laryngeal cancer report group, 1991; Lefebvre et al, 1996, 2004), establishing sequential treatment as a standard approach. At effectually the same fourth dimension, CRT emerged every bit a highly efficacious handling and a large Intergroup trial was therefore initiated. Intergroup trial 91–xi had as a primary finish point larynx preservation in resectable patients with stage 3 or IV (just excluding T4) illness (Table 3) (Forastiere et al, 2003). The three treatment arms compared sequential chemotherapy and radiation, concurrent CRT, and radiotherapy alone. Laryngectomy was reserved for patients who did non respond to induction chemotherapy or with residual or recurrent disease following completion of radiotherapy on all iii artillery. Laryngeal preservation, the primary end point of this trial, was superior in the concomitant CRT arm compared to both sequential CRT (88 vs 75% 2-yr laryngeal preservation rate, P=0.005) and radiotherapy solitary (88 vs 70% 2-yr laryngeal preservation, P<0.001). On the other manus, astute high-grade toxicities were more common in both artillery involving chemotherapy. Similar to other CRT trials, late toxicities and swallowing part at 2 years were equivalent between the three artillery. As a secondary outcome, CRT achieved the highest locoregional control rate. Survival was not significantly dissimilar between all 3 treatment arms, while both chemotherapy artillery showed lower distant failure rates compared to radiotherapy lonely. In conclusion concurrent CRT should be considered standard of intendance in this patient population. Sequential CRT is not more than efficacious, simply more toxic than radiotherapy, and should therefore not be routinely used.
In other anatomical locations, no comparable level i evidence for CRT exists. The earlier EORTC 24891 trial (Lefebvre et al, 1996, 2004) included hypopharynx tumours just merely used sequential CRT. Even so, based on the superior locoregional command rates in comparison to radiotherapy alone in locally advanced unresectable patients, CRT is likely to be at the very to the lowest degree a reasonable choice for organ preservation in full general.
An evolving role for consecration chemotherapy
The administration of induction chemotherapy prior to definitive local therapy remains controversial. The involvement in systemically active chemotherapy arose from the observation that, with highly effective local control measures, the majority of patients, who failed therapy, would recur at a distant site. This was presumably from micrometastatic disease that local therapy or lower dose chemotherapy as function of chemoradiation would non adequately treat. This theoretical statement has been tested in several trials that have been unable to show a consequent survival do good (1987; Schuller et al, 1988; 1991; Paccagnella et al, 1994; El-Sayed and Nelson 1996; Domenge et al, 2000; Pignon et al, 2000). (Tabular array 4a). Design issues as well as concerns that less effective local therapy in the past may have decreased the power to bear witness a survival benefit, remain. Nevertheless, induction chemotherapy continues to be an ongoing surface area of research with several centres around the globe continuing to investigate this approach, which can produce CR rates in 30–65% of patients and overall response rates of 70–85% (Hitt et al, 2003; Knecht et al, 2003; Knecht et al, 2003; Gustin et al, 2004; Nadeem et al, 2004; Vermorken et al, 2004).
Ii European studies have shown evidence of a survival do good with induction chemotherapy: the Italian GSTTC report (Gruppo di Studio sui Tumori della Testa) (Domenge et al, 2000) demonstrated increased cure rates in a subset of nonoperable patients and the French GETTEC trial (Groupe d'Etude des Tumeurs de la Tete et du Cou), which was closed early (Paccagnella et al, 1994). A large meta-analysis past Pignon et al (2000) furthermore showed a 5% increase in survival only for trials using a cisplatin/fluorouracil (v-FU) combination, reaching statistical significance (P=0.05).
More than recently, two trials compared a triplet combination of a taxane (docetaxel or paclitaxel), cisplatin, and 5-FU (TPF) with doublet cisplatin/5-FU (PF) (Hitt et al, 2003; Vermorken et al, 2004). Consistent in both randomised trials, TPF was well tolerated and significantly more effective in detail in the recently reported EORTC 24971 trial past Vermorken et al (2004), where a significant difference in overall survival was seen. The trials are not fully published, but the data from both trials are consequent suggesting that a triplet combination (TPF) including a taxane has potential to sally as a standard choice for induction chemotherapy in the futurity.
Still induction chemotherapy cannot be considered standard of care due to the lack of convincing phase III evidence, only lower level show suggests that it is reasonably safe and may benefit patients at high risk for distal failure as indicated by advanced nodal involvement. The triplet combination of a taxane, cisplatin, and 5-FU seems to have a high degree of activity and acceptable toxicity (Table 4b) (Hitt et al, 2003; Knecht et al, 2003; Vermorken et al, 2004).
Induction chemotherapy and concomitant CRT
The combination of induction chemotherapy and concomitant CRT appears to be of detail involvement due to their complementary effects with the erstwhile leading to a reduction of afar disease and the latter achieving locoregional control. Results of induction chemotherapy followed by CRT are encouraging (Hainsworth et al, 2002; Machtay et al, 2002; Haraf et al, 2003; Vokes et al, 2003) (Table 4b) and propose a reasonable toxicity profile, lower distal failure rates, and improved survival in comparison with historical controls using the same CRT regimen (Vokes et al, 2003).
In summary, there is a possibility that consecration chemotherapy may improve survival in locally advanced HNSCC in combination with CRT every bit suggested by lower level evidence. Adequate phase III show is non available currently, and until farther data become available, induction chemotherapy should not be used routinely in HNSCC exterior a clinical trial, just may be appropriate given its well-established safety profile in high-risk situations.
Epidermal growth factor receptor inhibition in combination with radiotherapy or CRT
The EGF receptor as well every bit its primary ligand TGF-α are expressed in approximately 80% of HNSCC (Grandis et al, 1996; Grandis et al, 1998) and play an important role in the biology of this disease (Barrandon and Greenish 1987; Grandis et al, 1998; O-charoenrat et al, 2000; Veikkola et al, 2000; Ang et al, 2002). A multitude of agents inhibiting EGFR are in various stages of evolution and encouraging unmarried agent activity has been reported in recurrent or metastatic HNSCC (Cohen EE et al, 2003; Soulieres et al, 2004). Despite the loftier efficacy of electric current treatments, EGFR inhibitors hold hope in 2 important ways: (ane) to further improve efficacy for patients at take chances for recurrence and (2) to decrease treatment-related toxicities by replacing more toxic cytotoxic drugs without jeopardising survival.
A phase III multicentre trial (Bonner et al, 2004) enrolled 424 patients with stage III/Iv HNSCC randomising them to receive radiotherapy with or without concomitant cetuximab, a humanised monoclonal antibiotic directed against the EGFR, as a main treatment. Overall, the regimen was well tolerated, with an increase in class iii cutaneous toxicity from xviii to 34% and some mild increases in allergic reactions. Even though only preliminary data are available, a marked increase in median overall survival from 28 months with radiations alone to 54 months with the add-on of cetuximab was reported. Locoregional command was significantly improved in the cetuximab arm as well (48 vs 56% at 2 years, P=0.02).
These reports are very encouraging; however, the major difficulty in interpreting and using these data is that it remains unclear how radiotherapy plus cetuximab compares to CRT as the reported trial used a control arm that would be considered inferior in light of contempo evidence discussed above. Given the immature nature of the data and in item the disability to compare to electric current standards of intendance, it is prudent to expect until further information becomes bachelor. Still it is reasonable to consider this regimen in patients with poor functioning status, who are not good candidates for CRT or surgery.
Another written report of the combination of gefitinib with CRT (5-FU, hydroxyurea, and twice daily radiotherapy), following induction chemotherapy and followed by gefitinib maintenance, provided insights into a potential role for EGFR inhibitors (Cohen EW et al, 2004). In comparison to previous similarly designed trials, this treatment was at least as efficacious as a comparable taxane-containing regimen (paclitaxel, 5-FU, hydroxyurea, and radiotherapy) (Vokes et al, 1999) and meliorate tolerated. Some other trial exploring the combination of gefitinib and radiation with or without cisplatin is currently ongoing and demonstrates that the combination of gefitinib with cisplatin and radiotherapy appears feasible.
Conclusion
Treatment for locally advanced HNSCC has improved dramatically during the past decade, allowing a word of cure in many patients, and an prove-based algorithm to guide treatment can be created (Effigy i). In detail, CRT has established itself equally a central treatment modality either upfront equally definitive therapy or every bit an adjuvant to surgery, due to its excellent local control rates, increased survival, and college rates of organ preservation. For the future, the integration of EGFR inhibitors is poised to play an increasingly important curative role while potentially decreasing toxicity. Still it may be even more of import to consolidate our current knowledge and abilities and enforce quality standards to allow as many patients as possible to benefit from already first-class handling approaches.
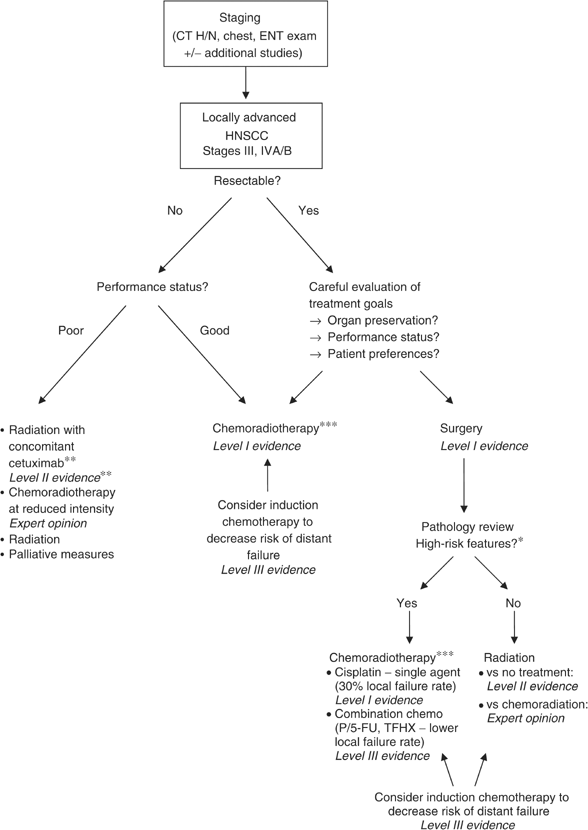
Evidence-based treatment algorithm for direction of locally avant-garde HNSCC.

Change history
-
xvi November 2011
This newspaper was modified 12 months later initial publication to switch to Creative Commons licence terms, as noted at publication
References
-
Adelstein DJ, Li Y, Adams GL, Wagner Jr H, Kish JA, Ensley JF, Schuller DE, Forastiere AA (2003) An intergroup phase III comparing of standard radiations therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol 21(1): 92–98
-
Adelstein DJ, Saxton JP, Lavertu P, Rybicki LA, Esclamado RM, Woods BG, Strome 1000, Carroll MA (2002) Maximizing local command and organ preservation in stage Iv squamous cell caput and neck cancer with hyperfractionated radiation and concurrent chemotherapy. J Clin Oncol 20(5): 1405–1410
-
Ang KK, Berkey BA, Tu X, Zhang HZ, Katz R, Hammond EH, Fu KK, Milas Fifty (2002) Touch on of epidermal growth factor receptor expression on survival and design of relapse in patients with avant-garde caput and neck carcinoma. Cancer Res 62(24): 7350–7356
-
Argiris A, Stenson KM, Brockstein Be, Mittal BB, Pelzer H, Kies MS, Jayaram P, Portugal Fifty, Wenig BL, Rosen FR, Haraf DJ, Vokes EE (2004) Neck autopsy in the combined-modality therapy of patients with locoregionally advanced head and neck cancer. Caput Neck 26(five): 447–455
-
Bachaud JM, Cohen-Jonathan Eastward, Alzieu C, David JM, Serrano E, Daly-Schveitzer N (1996) Combined postoperative radiotherapy and weekly cisplatin infusion for locally avant-garde head and cervix carcinoma: final report of a randomized trial. Int J Radiat Oncol Biol Phys 36(5): 999–1004
-
Barrandon Y, Green H (1987) Jail cell migration is essential for sustained growth of keratinocyte colonies: the roles of transforming growth factor-alpha and epidermal growth gene. Cell fifty(7): 1131–1137
-
Benasso Grand, Bonelli L, Numico G, Corvo R, Sanguineti Yard, Rosso R, Vitale V, Merlano G (1997) Treatment with cisplatin and fluorouracil alternating with radiation favourably affects prognosis of inoperable squamous prison cell carcinoma of the caput and neck: results of a multivariate analysis on 273 patients. Ann Oncol 8(8): 773–779
-
Bernier J, Domenge C, Ozsahin Chiliad, Matuszewska K, Lefebvre JL, Greiner RH, Giralt J, Maingon P, Rolland F, Bolla M, Cognetti F, Bourhis J, Kirkpatrick A, van Glabbeke M (2004) Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. North Engl J Med 350(19): 1945–1952
-
Bonner JA, Giralt J, Harari PM, Cohen R, Jones C, Sur RK, Rabin D, Azarnia North, Needle MN, Ang KK (2004) 'Cetuximab prolongs survival in patients with locoregionally advanced squamous cell carcinoma of head and neck: a phase III study of high dose radiations therapy with or without cetuximab'. J Clin Oncol, 2004 ASCO Annual Meeting Proceedings (Post-Meeting Edition) 22 14S (July 15 Supplement): 5507
-
Brizel DM, Albers ME, Fisher SR, Scher RL, Richtsmeier WJ, Hars V, George SL, Huang AT, Prosnitz LR (1998) Hyperfractionated irradiation with or without concurrent chemotherapy for locally advanced head and cervix cancer. N Engl J Med 338(25): 1798–1804
-
Cohen EE, Rosen F, Stadler WM, Recant Due west, Stenson K, Huo D, Vokes EE (2003) Phase Ii trial of ZD1839 in recurrent or metastatic squamous jail cell carcinoma of the head and neck. J Clin Oncol 21(10): 1980–1987
-
Cohen EW et al. (2004) 'Phase II trial of induction chemotherapy followed by gefitinib based chemoradiation and adjuvant therapy in patients with locally advanced Head and Neck Cancer'. The Tertiary International Chicago Symposium on Malignacies of the Chest and Head and Neck. Chicago, IL
-
Cooper JS, Pajak TF, Forastiere A, Jacobs J, Fu KK, Ang KK, Laramore GE, Al-Sarraf Thousand (1998) Precisely defining high-run a risk operable head and neck tumors based on RTOG #85-03 and #88-24: targets for postoperative radiochemotherapy? Caput Neck xx(7): 588–594
-
Cooper JS, Pajak TF, Forastiere AA, Jacobs J, Campbell BH, Saxman SB, Kish JA, Kim HE, Cmelak AJ, Rotman G, Machtay Yard, Ensley JF, Chao KS, Schultz CJ, Lee N, Fu KK (2004) Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-prison cell carcinoma of the head and neck. N Engl J Med 350(19): 1937–1944
-
Domenge C, Loma C, Lefebvre JL, De Raucourt D, Rhein B, Wibault P, Marandas P, Coche-Dequeant B, Stromboni-Luboinski K, Sancho-Garnier H, Luboinski B (2000) Randomized trial of neoadjuvant chemotherapy in oropharyngeal carcinoma. French Groupe d' Etude des Tumeurs de la Tete et du Cou (GETTEC)'. Br J Cancer 83(12): 1594–1598
-
El-Sayed Due south, Nelson North (1996) Adjuvant and adjunctive chemotherapy in the direction of squamous cell carcinoma of the head and neck region. A meta-assay of prospective and randomized trials. J Clin Oncol fourteen(3): 838–847
-
Fletcher GH, Evers WT (1970) Radiotherapeutic management of surgical recurrences and postoperative residuals in tumors of the head and neck. Radiology 95(1): 185–188
-
Forastiere AA, Goepfert H, Maor G, Pajak TF, Weber R, Morrison W, Glisson B, Trotti A, Ridge JA, Chao C, Peters G, Lee DJ, Leaf A, Ensley J, Cooper J (2003) Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. North Engl J Med 349(22): 2091–2098
-
Grandis JR, Melhem MF, Barnes EL, Tweardy DJ (1996) Quantitative immunohistochemical analysis of transforming growth factor-alpha and epidermal growth factor receptor in patients with squamous prison cell carcinoma of the head and neck. Cancer 78(6): 1284–1292
-
Grandis JR, Melhem MF, Gooding WE, Day R, Holst VA, Wagener MM, Drenning SD, Tweardy DJ (1998) Levels of TGF-blastoff and EGFR protein in head and neck squamous cell carcinoma and patient survival. J Natl Cancer Inst 90(11): 824–832
-
Gustin D, Haraf DJ, Stenson Grand, Cohen East, Rosen F, Argiris A, Brockstein Be, Witt ME, Dekker A, Vokes EE (2004) 'Treatment of avant-garde head and neck cancer with consecration chemotherapy followed by chemoradiotherapy with reduced radiation dose'. J Clin Oncol, 2004 ASCO Annual Meeting Proceedings (Mail-Meeting Edition) 22 14S (July fifteen Supplement): 5546
-
Hainsworth JD, Meluch AA, McClurkan Due south, Gray JR, Stroup SL, Burris HA 3rd, Yardley DA, Bradof JE, Yost Chiliad, Ellis JK, Greco FA (2002) Induction paclitaxel, carboplatin, and infusional 5-FU followed by concurrent radiations therapy and weekly paclitaxel/carboplatin in the treatment of locally advanced head and neck cancer: a phase Two trial of the Minnie Pearl Cancer Research Network. Cancer J eight(iv): 311–321
-
Haraf DJ, Rosen FR, Stenson K, Argiris A, Mittal BB, Witt ME, Brockstein BE, List MA, Portugal L, Pelzer H, Weichselbaum RR, Vokes EE (2003) Induction chemotherapy followed past concomitant TFHX chemoradiotherapy with reduced dose radiation in advanced caput and neck cancer. Clin Cancer Res ix(xvi Part 1): 5936–5943
-
Head & Neck Contracts Program (1987) Adjuvant chemotherapy for advanced caput and cervix squamous carcinoma. Final written report of the Caput and Neck Contracts Program. Cancer 60(3): 301–311
-
Hitt R, Lopez-Pousa A, Rodriguez M, Martinez-Trufero J, Cervantes A, Carles J, Lopez E, Pena C, Miquel Q, Paz-Ares 50 (2003) 'Phase III study comparison cisplatin (P) & 5-fluoruracil (F) versus P, F and paclitaxel (T) every bit induction therapy in locally avant-garde head & neck cancer (LAHNC)'. Proc Am Soc Clin Oncol 22: 496 (abstr 1997)
-
Hoffman H, Cooper JS, Weber R, Ang One thousand, Porter K, Langer CJ (2004) 'Changing patterns of exercise in the management of nasopharynx carcinoma (NPC): analysis of the National Cancer Database (NCDB)'. J Clin Oncol, 2004 ASCO Annual Meeting Proceedings (Mail service-Meeting Edition) 22 14S (July 15 Supplement): 5507
-
Huang DT, Johnson CR, Schmidt-Ullrich R, Grimes M (1992) Postoperative radiotherapy in head and neck carcinoma with extracapsular lymph node extension and/or positive resection margins: a comparative written report. Int J Radiat Oncol Biol Phys 23(4): 737–742
-
Jeremic B, Shibamoto Y, Milicic B, Nikolic North, Dagovic A, Aleksandrovic J, Vaskovic Z, Tadic 50 (2000) Hyperfractionated radiations therapy with or without concurrent low-dose daily cisplatin in locally advanced squamous jail cell carcinoma of the head and neck: a prospective randomized trial. J Clin Oncol 18(7): 1458–1464
-
Kies MS, Haraf DJ, Rosen F, Stenson G, List K, Brockstein B, Chung T, Mittal BB, Pelzer H, Portugal L, Rademaker A, Weichselbaum R, Vokes EE (2001) Concomitant infusional paclitaxel and fluorouracil, oral hydroxyurea, and hyperfractionated radiation for locally advanced squamous head and neck cancer. J Clin Oncol 19(seven): 1961–1969
-
Knecht R, Baghi M, Hambek M, Tesch H, Gstottner Due west (2003) 'Response rate and outcome of a novel induction chemotherapy regimen (TPF) in the first-line therapy of avant-garde caput and neck carcinomas (SCCHN)'. Proc Am Soc Clin Oncol 22: 501 (abstr 2017)
-
Kramer Southward, Gelber RD, Snow JB, Marcial VA, Lowry LD, Davis LW, Chandler R (1987) Combined radiations therapy and surgery in the management of avant-garde head and neck cancer: final report of study 73-03 of the Radiation Therapy Oncology Group. Caput Cervix Surg 10(i): 19–30
-
Laramore GE, Scott CB, al-Sarraf M, Haselow RE, Ervin TJ, Wheeler R, Jacobs JR, Schuller DE, Gahbauer RA, Schwade JG et al. (1992) Adjuvant chemotherapy for resectable squamous prison cell carcinomas of the head and neck: report on Intergroup Study 0034. Int J Radiat Oncol Biol Phys 23(4): 705–713
-
Lavertu P, Adelstein DJ, Saxton JP, Secic K, Wanamaker JR, Eliachar I, Woods BG, Strome M (1997) Management of the neck in a randomized trial comparing concurrent chemotherapy and radiotherapy with radiotherapy lonely in resectable stage 3 and IV squamous cell head and cervix cancer. Caput Neck nineteen(7): 559–566
-
Lefebvre JL, Chevalier D, Luboinski B, Kirkpatrick A, Collette 50, Sahmoud T (1996) Larynx preservation in pyriform sinus cancer: preliminary results of a European Organization for Inquiry and Handling of Cancer stage III trial. EORTC Caput and Neck Cancer Cooperative Grouping. J Natl Cancer Inst 88(13): 890–899
-
Lefebvre JL, Chevalier D, Luboinski B, Traissac L, Andry G, De Raucourt D, Collette L, Bernier J (2004) 'Is laryngeal preservation (LP) with induction chemotherapy (ICT) condom in the handling of hypopharyngeal SCC? Concluding results of the phase Three EORTC 24891 trial'. J Clin Oncol, 2004 ASCO Annual Coming together Proceedings (Post-Coming together Edition) 22 14S (July 15 Supplement): 5531
-
Machtay K, Rosenthal DI, Hershock D, Jones H, Williamson Due south, Greenberg MJ, Weinstein GS, Aviles VM, Chalian AA, Weber RS (2002) Organ preservation therapy using induction plus concurrent chemoradiation for avant-garde resectable oropharyngeal carcinoma: a University of Pennsylvania Phase Ii Trial. J Clin Oncol 20(19): 3964–3971
-
Milano MT, Haraf DJ, Stenson KM, Witt ME, Eng C, Mittal BB, Argiris A, Pelzer H, Kozloff MF, Vokes EE (2004) Phase I study of concomitant chemoradiotherapy with paclitaxel, fluorouracil, gemcitabine, and twice-daily radiations in patients with poor-prognosis cancer of the caput and neck. Clin Cancer Res 10(xv): 4922–4932
-
Nadeem A, Desai S, Chougule P, Wanebo H, Ruhl C, Joyce D, Tarro J, Kennedy T, Ready N (2004) 'Decreased distant recurrence and preserved local control using dose dumbo consecration weekly paclitaxel (P) and carboplatin (C) followed past concurrent paclitaxel, carboplatin and radiotherapy (CRT) in locally advanced head and neck squamous cell cancers (HN-SCC)'. J Clin Oncol, 2004 ASCO Annual Coming together Proceedings (Mail service-Meeting Edition) 22 14S (July 15 Supplement): 5545
-
O-charoenrat P, Modjtahedi H, Rhys-Evans P, Court WJ, Box GM, Eccles SA (2000) Epidermal growth factor-similar ligands differentially upward-regulate matrix metalloproteinase 9 in head and neck squamous carcinoma cells. Cancer Res 60(iv): 1121–1128
-
Paccagnella A, Orlando A, Marchiori C, Zorat PL, Cavaniglia G, Sileni VC, Jirillo A, Tomio L, Fila G, Fede A et al. (1994) Phase 3 trial of initial chemotherapy in stage III or Four head and neck cancers: a study by the Gruppo di Studio sui Tumori della Testa eastward del Collo. J Natl Cancer Inst 86(4): 265–272
-
Parkin DM, Bray F, Ferlay J, Pisani P (2001) Estimating the world cancer burden: Globocan 2000. Int J Cancer 94(ii): 153–156
-
Pignon JP, Bourhis J, Domenge C, Designe L (2000) Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: iii meta-analyses of updated individual data. MACH-NC Collaborative Grouping. Meta-Analysis of Chemotherapy on Caput and Neck Cancer. Lancet 355(9208): 949–955
-
Robbins KT, Medina JE, Wolfe GT, Levine PA, Sessions RB, Pruet CW (1991) Standardizing cervix dissection terminology. Official report of the Academy's Committee for Head and Neck Surgery and Oncology'. Arch Otolaryngol Caput Cervix Surg 117(6): 601–605
-
Sankaranarayanan R, Masuyer Due east, Swaminathan R, Ferlay J, Whelan Southward (1998) Head and cervix cancer: a global perspective on epidemiology and prognosis. Anticancer Res 18(6B): 4779–4786
-
Schuller DE, Metch B, Stein DW, Mattox D, McCracken JD (1988) Preoperative chemotherapy in avant-garde resectable head and neck cancer: final report of the Southwest Oncology Group. Laryngoscope 98(11): 1205–1211
-
Snow GB, Annyas AA, van Slooten EA, Bartelink H, Hart AA (1982) Prognostic factors of neck node metastasis. Clin Otolaryngol 7(3): 185–192
-
Soulieres D, Senzer NN, Vokes EE, Hidalgo Yard, Agarwala SS, Siu LL (2004) Multicenter Phase II report of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and cervix. J Clin Oncol 22(one): 77–85
-
Stenson KM, Haraf DJ, Pelzer H, Recant W, Kies MS, Weichselbaum RR, Vokes EE (2000) The role of cervical lymphadenectomy afterwards aggressive concomitant chemoradiotherapy: the feasibility of selective cervix dissection. Arch Otolaryngol Head Neck Surg 126(8): 950–956
-
VA Laryngeal cancer study group (1991) Induction chemotherapy plus radiations compared with surgery plus radiation in patients with avant-garde laryngeal cancer. The Department of Veterans Affairs Laryngeal Cancer Study Grouping. N Engl J Med 324(24): 1685–1690
-
Veikkola T, Karkkainen M, Claesson-Welsh 50, Alitalo K (2000) Regulation of angiogenesis via vascular endothelial growth factor receptors. Cancer Res sixty(2): 203–212
-
Vermorken JB, Remenar E, Van Herpen C, Germa Lluch J, Stewart Southward, Gorlia T, Degardin One thousand, Schollen K, Bernier J (2004) 'Standard cisplatin/infusional 5-fluorouracil (PF) vs docetaxel (T) plus PF (TPF) as neoadjuvant chemotherapy for nonresectable locally advanced squamous cell carcinoma of the head and cervix (LA-SCCHN): a phase III trial of the EORTC Head and Neck Cancer Group (EORTC #24971)'. J Clin Oncol, 2004 ASCO Annual Coming together Proceedings (Mail-Coming together Edition) 22 14S (July 15 Supplement): 5508
-
Vikram B, Strong EW, Shah JP, Spiro R (1984) Failure at the master site post-obit multimodality treatment in advanced caput and neck cancer. Caput Neck Surg 6(iii): 720–723
-
Vokes EE, Haraf DJ, Brockstein BE, Weichselbaum RR (1999) Paclitaxel, 5-fluorouracil, hydroxyurea, and concomitant radiation therapy for poor-prognosis head and neck cancer. Semin Radiat Oncol ix(2 Suppl 1): 70–76
-
Vokes EE, Stenson K, Rosen FR, Kies MS, Rademaker AW, Witt ME, Brockstein BE, List MA, Fung BB, Portugal L, Mittal BB, Pelzer H, Weichselbaum RR, Haraf DJ (2003) Weekly carboplatin and paclitaxel followed by concomitant paclitaxel, fluorouracil, and hydroxyurea chemoradiotherapy: curative and organ-preserving therapy for advanced head and cervix cancer. J Clin Oncol 21(2): 320–326
-
Vokes EE, Weichselbaum RR, Lippman SM, Hong WK (1993) Head and neck cancer. N Engl J Med 328(3): 184–194
Acknowledgements
We give thanks Michelle Scheuer for help in training of the manuscript.
Writer information
Affiliations
Respective writer
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Akin three.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/past-nc-sa/three.0/
Reprints and Permissions
Well-nigh this article
Cite this commodity
Seiwert, T., Cohen, E. State-of-the-art management of locally advanced head and neck cancer. Br J Cancer 92, 1341–1348 (2005). https://doi.org/10.1038/sj.bjc.6602510
-
Received:
-
Accustomed:
-
Published:
-
Effect Engagement:
-
DOI : https://doi.org/10.1038/sj.bjc.6602510
Keywords
- head & cervix cancer
- chemoradiotheraphy
- squamous jail cell carcinoma (HNSCC)
- epidermal growth cistron receptor (EGFR)
Further reading
Source: https://www.nature.com/articles/6602510
0 Response to "Radiotherapy for Head and Neck Squamous Cell Carcinoma State of the Art and Future Directions"
Post a Comment